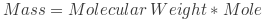
In chemistry, a mole is a unit of measurement that is used to express the amount of a substance. The mole is defined as the amount of a substance that contains the same number of particles (atoms, molecules, or ions) as there are in 12 grams of carbon-12. This number of particles is known as Avogadro's number, which is approximately 6.02 x 10^23.
The concept of the mole was first introduced by the Italian scientist Amedeo Avogadro in 1811, but it wasn't until the early 20th century that the mole became an official unit of measurement in chemistry.
The mole is important in chemistry because it allows to measure the amount of a substance on a macroscopic scale. In other words, it allows to measure the amount of a substance in grams or other units of mass, rather than on a microscopic scale using the number of particles.
For example, if you have 1 mole of water (H2O), you know that it contains 6.02 x 10^23 water molecules. If you have 2 moles of water, you know that it contains 2 times that number of molecules (12.04 x 10^23).
The mole is particularly useful when working with gases because gases are difficult to measure on a macroscopic scale. The volume of a gas depends on temperature and pressure, which can vary significantly. However, the number of particles in a gas is directly proportional to its volume, so measuring the number of particles using moles allows chemists to work with gases on a more macroscopic scale.
One mole of any substance has a mass equal to its atomic or molecular weight in grams. For example, one mole of carbon-12 atoms weighs 12 grams, while one mole of water (H2O) molecules weighs 18 grams (2 hydrogen atoms weigh 1 gram each, and 1 oxygen atom weighs 16 grams). This relationship between moles and mass is known as the molar mass.
The mole concept also plays an important role in the study of solution chemistry, where it is used to determine the concentration of a solution. Concentration is expressed as the amount of solute per unit volume of solution, and the amount of solute is typically measured in moles.