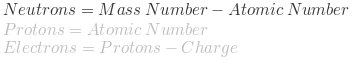
Neutrons are one of the three fundamental particles that make up an atom, along with protons and electrons. They are uncharged particles that reside in the nucleus of an atom, alongside positively charged protons. The number of neutrons in an atom can vary, and atoms of the same element with different numbers of neutrons are known as isotopes.
The discovery of the neutron was made in 1932 by British physicist James Chadwick. He found that the radiation emitted from beryllium when bombarded with alpha particles consisted of particles that were neutral in charge, unlike the positively charged alpha particles. He concluded that these neutral particles were a new type of fundamental particle and called them neutrons.
Neutrons have a mass of approximately 1.675×10−27 kg, which is slightly greater than the mass of protons. They are found in the nucleus of atoms alongside protons, which have a positive charge. Neutrons do not have an electric charge, which makes them unique among the particles found in the nucleus.
Neutrons are unstable when they are not bound in the nucleus, and they typically have a half-life of about 10 minutes. They can be produced in a variety of ways, including through the decay of radioactive isotopes and by bombarding atomic nuclei with high-energy particles.
Neutrons play a critical role in nuclear reactions, such as fission and fusion. In nuclear fission, a neutron is absorbed by a heavy nucleus, causing it to split into two smaller nuclei and releasing a large amount of energy in the form of heat and radiation. This process is used in nuclear reactors to produce electricity.
In nuclear fusion, two nuclei are combined to form a heavier nucleus, and again, a large amount of energy is released. Fusion is the process that powers the sun and other stars.
Neutrons interact with matter differently than other subatomic particles. Because they do not have an electric charge, they are not affected by electromagnetic forces. Instead, they interact with matter through a process known as the strong nuclear force, which is responsible for holding the nucleus of an atom together.
Neutrons can be absorbed by atomic nuclei, which can cause the nucleus to become unstable and decay. Neutrons can also be scattered or bounce off atomic nuclei, depending on the energy of the neutron and the atomic properties of the nucleus.
Neutrons have many important applications in science and technology. They are used in a variety of imaging techniques, including neutron radiography and neutron scattering, which can be used to study the structure of materials at the atomic level.
Neutrons are also used in the production of radioisotopes, which are used in medicine for diagnostic imaging and cancer treatment. They are also used in the analysis of archaeological and historical artifacts, as well as in the study of geological and environmental samples.